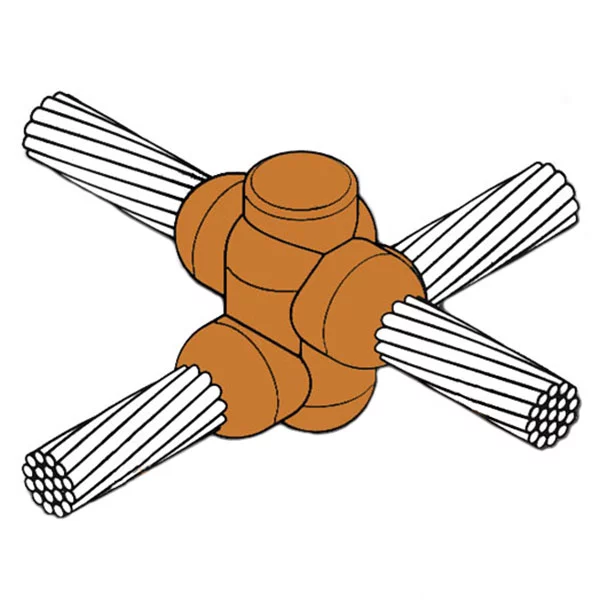
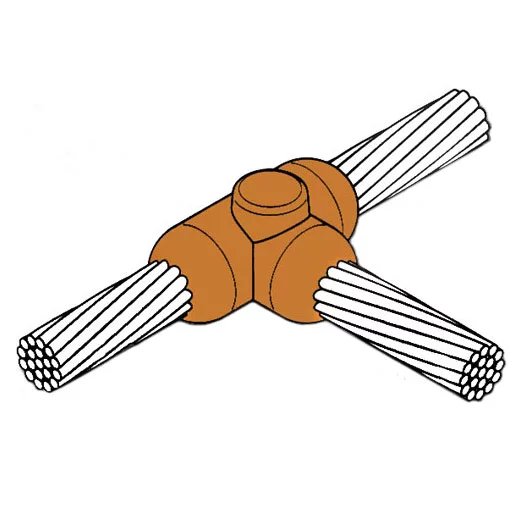
Introduction to Exothermic Welding
Introduction
“Exothermic” is a chemical term used to describe a reaction that produces heat.
Exothermic welding, also known as “thermit welding” or “aluminothermic welding” is a welding process for permanently joining materials (usually copper conductors) that employs an exothermic reaction. The exothermic reaction requires no external heat or a power source. All that is required is a spark to initiate the reaction.
The exothermic reaction occurs between copper oxide and aluminium powder (contained within the weld metal) creating molten super-heated copper and an aluminium oxide slag. When an ignition spark comes into contact with the weld metal, it causes an exothermic reaction within the weld metal, melting and separating the metals. The aluminium rises to the top of the connection creating a slag leaving the molten copper to flow around the joint, creating the weld.
The KingsWeld exothermic connection is a permanent, maintenance-free weld that will not loosen overtime or deteriorate with age. The connections’ current carrying capability is equal to or greater than that of the conductors being joined. In other words, there is no increase in resistance in an exothermically welded connection, unlike in most pressure type (bolt/crimp) connections.
Throughout the world, exothermic welding process has been shown to be the best choice where safety, reliability, current carrying capacity and longevity are critical.
Advantages
- The current carrying capacity of the connection is greater than or equal to that of the conductor
- Has a lower electrical resistance than a mechanical connection
- Does not deteriorate with age
- Does not loosen over time
- Can withstand repeated high current surges without deterioration
- Does not require an external power source
- Used to weld copper, copper alloys, copper bonded steel, various steel alloys, including stainless steel
- Quick and easy to install
- Exceptional corrosion resistance due a very high copper content (97%+)
- Fusion temperature is in excess of 2000°C forming a molecular bond
This adds up to a superior connection when compared to mechanical or pressure type (crimp) connectors.
This adds up to a superior connection when compared to mechanical or pressure type (crimp) connectors.

The KingsWeld Process
The KingsWeld exothermic welding process is a simple, self-contained, efficient way of welding copper-to-copper or copper-to-steel.
Each connection uses a KingsWeld weld metal which, when ignited, creates an exothermic reaction between copper oxide and aluminium powder.
The connections are produced inside a graphite mould, specifically designed to suit the size of conductors to be welded as well as the specific joint configuration.
Each exothermic connection requires a specific mould designed to suit the joint configuration and conductors being used. Each mould type requires a specific weld metal size. This can be found in our weld metal selection table.
Once the correct mould and weld metal have been selected, the process is simple and straightforward.
The conductors are positioned in the graphite mould. A steel retaining disc is then inserted into the mould before any weld metal is added. Only after the disc is in place and properly seated can the main weld metal (under the green cap) be poured into the reaction crucible. The ignition temperature of the main weld metal is approximately 1000°C. This is difficult to achieve and so we use a starter powder to start the exothermic reaction, this is contained under the red cap. The starter powder is similar to the main weld metal, but finer, allowing ignition at around 450°C (through using the spark from a flint ignitor).
The resultant exothermic reaction produces high temperature molten copper (in excess of 2000°C) and aluminium slag.
The molten copper melts the steel retaining disc and flows down the tap hole into the joint cavity. In doing so, the molten copper melts and welds the conductors into a solid homogenous joint.
The whole process takes no more than a few seconds. The aluminium oxide produced stays on top of the joint and is easily removed.